
Chemistry, 18.03.2020 20:29 anthonybowie99
5. At 20°C, the water autoionization constant, Kw, is 6.8 ´ 10–15. What is the H3O+ concentration in neutral water at this temperature? A. 6.8 × 10–7 M B. 3.4 × 10–15 M C. 6.8 × 10–15 M D. 8.2 × 10–8 M E. 1.0 × 10–7 M

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
5. At 20°C, the water autoionization constant, Kw, is 6.8 ´ 10–15. What is the H3O+ concentration in...
Questions


Social Studies, 02.04.2021 05:20


Mathematics, 02.04.2021 05:20

English, 02.04.2021 05:20

Mathematics, 02.04.2021 05:20

Mathematics, 02.04.2021 05:20

Mathematics, 02.04.2021 05:20




Mathematics, 02.04.2021 05:20

Computers and Technology, 02.04.2021 05:20


English, 02.04.2021 05:20

Mathematics, 02.04.2021 05:20

Mathematics, 02.04.2021 05:20

Mathematics, 02.04.2021 05:20

Mathematics, 02.04.2021 05:20

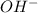 and
and 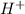 is equal to x. Then expression for
is equal to x. Then expression for 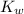 for the given reaction is as follows.
for the given reaction is as follows. ![K_{w} = [OH^{-}][H^{+}]](/tpl/images/0552/4456/ef741.png)
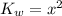
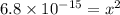
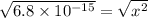
![[H^{+}] = 8.2 \times 10^{-8}](/tpl/images/0552/4456/07e73.png) M
M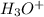 concentration in neutral water at this temperature is
concentration in neutral water at this temperature is 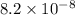 M.
M.

