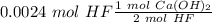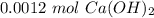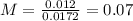Chemistry, 18.03.2020 21:34 odriskel49
An aqueous solution of calcium hydroxide is standardized by titration with a 0.164 M solution of hydroiodic acid. If 17.2 mL of base are required to neutralize 14.7 mL of the acid, what is the molarity of the calcium hydroxide solution?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1


Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
An aqueous solution of calcium hydroxide is standardized by titration with a 0.164 M solution of hyd...
Questions


Mathematics, 24.10.2019 02:30




English, 24.10.2019 02:30


Social Studies, 24.10.2019 02:30




Mathematics, 24.10.2019 02:30





History, 24.10.2019 02:30



Mathematics, 24.10.2019 02:30



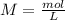
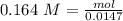
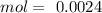
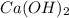 : 2 mol of
: 2 mol of 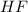 ). With this molar ratio, we can find the moles of
). With this molar ratio, we can find the moles of