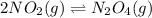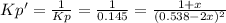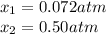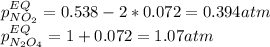Chemistry, 18.03.2020 21:59 briweaver9993
• Consider the reaction N2O4 (g) ⇌ 2 NO2 (g). At equilibrium, a 2.00-L reaction vessel contains NO2 at a pressure of 0.269 atm and N2O4 at a pressure of 0.500 atm. The reaction vessel is then compressed to 1.00 L. What will be the pressures of NO2 and N2O4 once equilibrium is re-established?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
• Consider the reaction N2O4 (g) ⇌ 2 NO2 (g). At equilibrium, a 2.00-L reaction vessel contains NO2...
Questions



History, 16.12.2020 23:30

Biology, 16.12.2020 23:30



Mathematics, 16.12.2020 23:30




Arts, 16.12.2020 23:30



Health, 16.12.2020 23:30


Biology, 16.12.2020 23:30

Mathematics, 16.12.2020 23:30



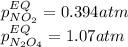
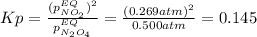
 is now present in order to take the reaction again to the equilibrium. Besides, the reaction changes as the products have more moles as:
is now present in order to take the reaction again to the equilibrium. Besides, the reaction changes as the products have more moles as: