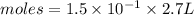Chemistry, 18.03.2020 23:01 AlwaysMarcus5577
For the reaction 2 H2O(g) equilibrium reaction arrow 2 H2(g) + O2(g), K = 2.4 ✕ 10−3 at a given temperature. At equilibrium in a 2.7 L container, it is found that [H2O(g)] = 2.0 ✕ 10−1 M and [H2(g)] = 2.5 ✕ 10−2 M. Calculate the moles of O2(g) present under these conditions.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1


Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
You know the right answer?
For the reaction 2 H2O(g) equilibrium reaction arrow 2 H2(g) + O2(g), K = 2.4 ✕ 10−3 at a given temp...
Questions




History, 28.07.2019 10:30





History, 28.07.2019 10:30

History, 28.07.2019 10:30

Mathematics, 28.07.2019 10:30

Mathematics, 28.07.2019 10:30








 moles of
moles of  will be produced.
will be produced.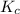 will be as follows,
will be as follows,![K_{c} = \frac{[H_{2} ]^2[O_{2} ]}{[H_{2}O ]^2 }](/tpl/images/0552/7877/22d2b.png)
![2.4\times10^{-2} = \frac{[2.5\times10^{-2} ]^2[O_{2} ]}{[2\times10^{-1} ]^2 }](/tpl/images/0552/7877/022e7.png)
![[O_{2} ]=\frac{[2.4\times10^{-3}][2\times10^{-1} ]^2 }{[2.5\times10^{-2} ]^2}](/tpl/images/0552/7877/7587e.png)
![[O_{2}]= 1.5\times10^{-1}M](/tpl/images/0552/7877/aba91.png)
![[O_{2} ]= \frac{moles}{LItre}](/tpl/images/0552/7877/7e5ff.png)
![[1.5\times10^{-1} ]= \frac{moles}{2.7}](/tpl/images/0552/7877/f2d4f.png)