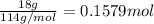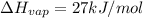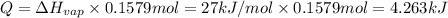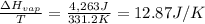Chemistry, 19.03.2020 00:24 hannahkharel2
Calculate the entropy change (J/K) for the vaporization of 18 g of a hydrocarbon (114 g/mole]), at its boiling point of 58.2°C. The enthalpy of vaporization of this hydrocarbon is 27 kJ/mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
Calculate the entropy change (J/K) for the vaporization of 18 g of a hydrocarbon (114 g/mole]), at i...
Questions



Computers and Technology, 17.02.2021 06:00

Mathematics, 17.02.2021 06:00


Mathematics, 17.02.2021 06:00

English, 17.02.2021 06:00

English, 17.02.2021 06:00





English, 17.02.2021 06:00



Mathematics, 17.02.2021 06:00



Mathematics, 17.02.2021 06:00

English, 17.02.2021 06:00