

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1


Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
The formula for Epsom salts is MgSO4*7H2O. If 1.250g of the compound is dissolved in water, calculat...
Questions

Advanced Placement (AP), 19.05.2020 02:11


Mathematics, 19.05.2020 02:11

Chemistry, 19.05.2020 02:11

Social Studies, 19.05.2020 02:11



History, 19.05.2020 02:11

Geography, 19.05.2020 02:11

History, 19.05.2020 02:11

Mathematics, 19.05.2020 02:11

Mathematics, 19.05.2020 02:11





Mathematics, 19.05.2020 02:11


Biology, 19.05.2020 02:11


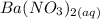 to precipitate barium sulfate.
to precipitate barium sulfate.

