
Chemistry, 19.03.2020 00:41 allieballey0727
A generic metal thiocyanate, M(SCN)2, has a Ksp value of 2.00×10−5. Calculate the molar solubility of the metal thiocyanate in 0.421 M KSCN. Express your answer numerically in units of mM to 4 decimal places.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
A generic metal thiocyanate, M(SCN)2, has a Ksp value of 2.00×10−5. Calculate the molar solubility o...
Questions



Computers and Technology, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57

History, 12.06.2020 07:57


Mathematics, 12.06.2020 07:57



History, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57





Biology, 12.06.2020 07:57



Mathematics, 12.06.2020 07:57

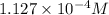 .
.![[SCN^-]= 0.421 M](/tpl/images/0553/0411/3a7d7.png)
![[M^{2+}]= ?](/tpl/images/0553/0411/05fb9.png)
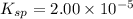
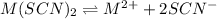
![K_{sp}=[M^{2+}]\times [SCN^-]^2](/tpl/images/0553/0411/0d18b.png)
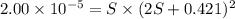
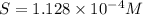
![[M^{2+}]=\frac{2.00\times 10^{-5}}{(0.421 M)^2}=1.127\times 10^{-4} M](/tpl/images/0553/0411/8e36e.png)


