Chemistry, 19.03.2020 00:53 dollazant2
A student has 540.0 mL of a 0.1035 M aqueous solution of Na2CrO4 to use in an experiment. She accidentally leaves the container uncovered and comes back the next week to find only a solid residue. The mass of the residue is 19.12 g. Determine the chemical formula of this residue.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 06:20
What is the magnitude of the force of gravity between to 1000 kg cars which are separated by distance of 25. 0 km on an interstate highway? the force between the two cars will be what
Answers: 3


Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
A student has 540.0 mL of a 0.1035 M aqueous solution of Na2CrO4 to use in an experiment. She accide...
Questions




Mathematics, 14.01.2021 20:20

Mathematics, 14.01.2021 20:20

Mathematics, 14.01.2021 20:20

English, 14.01.2021 20:20

Engineering, 14.01.2021 20:20

Mathematics, 14.01.2021 20:20

Mathematics, 14.01.2021 20:20

Arts, 14.01.2021 20:20

History, 14.01.2021 20:20

Mathematics, 14.01.2021 20:20


Mathematics, 14.01.2021 20:20

English, 14.01.2021 20:20

Health, 14.01.2021 20:20

Chemistry, 14.01.2021 20:20

Mathematics, 14.01.2021 20:20

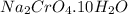
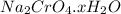
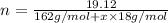
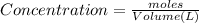
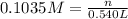
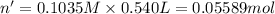
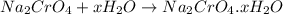
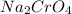 gives 1 mole of
gives 1 mole of