
Chemistry, 19.03.2020 01:08 ayoismeisjjjjuan
The Haber reaction for the manufacture of ammonia is: N2 + 3H2 → 2NH3 Without doing any experiments, which of the following can you say MUST be true? Disappearance rate of H2 = 3 (Disappearance rate of N2). The reaction is first order in N2. Reaction rate = -Δ[N2]/Δt. The reaction is not an elementary reaction. Δ[H2]/Δt will have a positive value. Disappearance rate of N2 = 3 (Disappearance rate of H2). The activation energy is positive.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1


You know the right answer?
The Haber reaction for the manufacture of ammonia is: N2 + 3H2 → 2NH3 Without doing any experiments,...
Questions


Physics, 23.06.2019 01:00




Mathematics, 23.06.2019 01:00


History, 23.06.2019 01:00




Physics, 23.06.2019 01:00







Mathematics, 23.06.2019 01:00

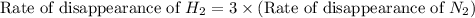
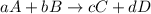
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0553/1420/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0553/1420/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0553/1420/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0553/1420/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0553/1420/d4b94.png)
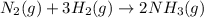
![\text{Rate of disappearance of }N_2=-\frac{d[N_2]}{dt}](/tpl/images/0553/1420/25b13.png)
![\text{Rate of disappearance of }H_2=-\frac{1}{3}\frac{d[H_2]}{dt}](/tpl/images/0553/1420/ebff2.png)
![\text{Rate of formation of }NH_3=+\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0553/1420/f55ec.png)


