
Chemistry, 19.03.2020 01:31 22katelynfrankouqqrb
One way to represent this equilibrium is: N2O4(g)2NO2(g) Indicate whether each of the following statements is true, T, or false, F. AT EQUILIBRIUM we can say that: 1. The concentration of NO2 is equal to the concentration of N2O4. 2. The rate of the dissociation of N2O4 is equal to the rate of formation of N2O4. 3. The rate constant for the forward reaction is equal to the rate constant of the reverse reaction. 4. The concentration of NO2 divided by the concentration of N2O4 is equal to a constant.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
One way to represent this equilibrium is: N2O4(g)2NO2(g) Indicate whether each of the following stat...
Questions



History, 09.04.2021 18:00




Mathematics, 09.04.2021 18:00




Mathematics, 09.04.2021 18:00

Mathematics, 09.04.2021 18:00


Mathematics, 09.04.2021 18:00

Physics, 09.04.2021 18:00




Mathematics, 09.04.2021 18:00

English, 09.04.2021 18:00

 is equal to the concentration of
is equal to the concentration of 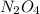 : False
: False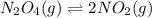
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0553/2027/271f5.png)


