
Consider a saturated aqueous solution of Ag2S that contains some excess solid Ag2S at the bottom. Which of the following statements is/are true? Adding AgNO3 to the solution will cause more Ag2S to dissolve. Adding some more Ag2S to the solution will cause more Ag2S to dissolve. Adding some water to the solution will cause more Ag2S to dissolve.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 16:00
The electron configuration for chromium is 1s22s22p63s23p63d54s1 instead of 1s22s22p63s23p63d44s1. the configuration is an exception to the pauli exclusion principle heisenberg uncertainty principle aufbau principle schrödinger equation
Answers: 3
You know the right answer?
Consider a saturated aqueous solution of Ag2S that contains some excess solid Ag2S at the bottom. Wh...
Questions

History, 12.01.2020 02:31

Mathematics, 12.01.2020 02:31







Mathematics, 12.01.2020 02:31

Biology, 12.01.2020 02:31


Mathematics, 12.01.2020 02:31

Mathematics, 12.01.2020 02:31

Mathematics, 12.01.2020 02:31

Mathematics, 12.01.2020 02:31



Mathematics, 12.01.2020 02:31


Mathematics, 12.01.2020 02:31

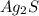 to dissolve is a true statement.
to dissolve is a true statement.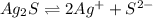
![K_{sp}=[Ag^{+}]^{2}[S^{2-}]](/tpl/images/0553/3669/0192a.png)
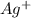 increases when
increases when 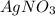 is added into solution. But value of
is added into solution. But value of 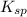 is constant at a certain temperature. Hence to keep
is constant at a certain temperature. Hence to keep 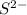 to produce more
to produce more 

