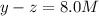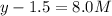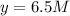Chemistry, 19.03.2020 03:07 DVM117x017
An initial mixture of nitrogen gas and hydrogen gas is reacted in a rigid container at a certain temperature by the reaction
3H2(g) +N2(g) <=> 2NH3(g)
At equilibrium the concentrations are [H2]=5.0 M, [N2]= 8.0M, [NH3]=3.0M
What were the concentrations of nitrogen gas and hydrogen gas that were reacted initially.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
An initial mixture of nitrogen gas and hydrogen gas is reacted in a rigid container at a certain tem...
Questions

Mathematics, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00

Chemistry, 02.06.2021 14:00


Mathematics, 02.06.2021 14:00


Computers and Technology, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00

Chemistry, 02.06.2021 14:00

English, 02.06.2021 14:00

Mathematics, 02.06.2021 14:00




Chemistry, 02.06.2021 14:00


English, 02.06.2021 14:00

English, 02.06.2021 14:00

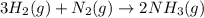
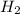 =
= 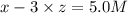
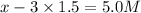
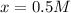
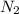 =
=