
Chemistry, 19.03.2020 04:08 maskythegamer
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in the atmosphere occur mainly in regions with large automobile or power plant emissions. The equilibrium constant for the reaction of N2 and 02 to give NO is very small. The reaction is, however, highly endothermic, with a heat of reaction equal to +180 kJ (Equation 7). N2(g) + O2(g) 180 kJ 2NO(g) Equation 7 +
(a) Use LeChâtelier's Principle to explain why the concentration of NO at equilibrium increases when the reaction takes place at high temperatures.
(b) Use LeChâtelier's Principle to predict whether the concentration of NO at equilibrium should increase when the reaction takes place at high pressures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in th...
Questions

History, 05.02.2021 17:30

Advanced Placement (AP), 05.02.2021 17:30

English, 05.02.2021 17:30

Mathematics, 05.02.2021 17:30

History, 05.02.2021 17:30



Mathematics, 05.02.2021 17:30

English, 05.02.2021 17:30

Health, 05.02.2021 17:30

Mathematics, 05.02.2021 17:30

Biology, 05.02.2021 17:30

History, 05.02.2021 17:30

Mathematics, 05.02.2021 17:30




Chemistry, 05.02.2021 17:30


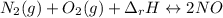
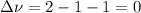
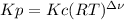
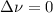 ,
, 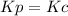 , so no effect in concentration is due to the pressure.
, so no effect in concentration is due to the pressure.

