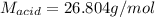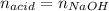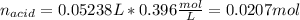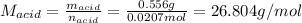Chemistry, 19.03.2020 07:52 quickestlearner6171
0.556 g of a solid white acid are dissolved in water and completely neutralized by the addition of 52.38 mL of 0.396 M NaOH. Calculate the molar mass of the acid, assuming it to be a monoprotic acid

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1


Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
0.556 g of a solid white acid are dissolved in water and completely neutralized by the addition of 5...
Questions




Arts, 15.04.2021 23:10

Mathematics, 15.04.2021 23:10

Mathematics, 15.04.2021 23:10

Physics, 15.04.2021 23:10







Mathematics, 15.04.2021 23:10

Mathematics, 15.04.2021 23:10




Mathematics, 15.04.2021 23:10

Mathematics, 15.04.2021 23:10