
A chemistry student adds a quantity of an unknown solid compound X to 750. mL of distilled water at 26.° C. After 10 minutes of stirring, all of the X has dissolved. The student then drains off the solution and evaporates the water under vacuum. A precipitate is left behind. The student washes, dries and weighs the precipitate. It weighs 0.012 kg.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1


Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2

You know the right answer?
A chemistry student adds a quantity of an unknown solid compound X to 750. mL of distilled water at...
Questions




English, 18.02.2021 03:20

English, 18.02.2021 03:20


Chemistry, 18.02.2021 03:20


Biology, 18.02.2021 03:20

Social Studies, 18.02.2021 03:20


Mathematics, 18.02.2021 03:20


Physics, 18.02.2021 03:20

Physics, 18.02.2021 03:20

Mathematics, 18.02.2021 03:20

Social Studies, 18.02.2021 03:20

Mathematics, 18.02.2021 03:20


History, 18.02.2021 03:20

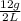
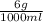
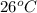 is 0.006 g/ml.
is 0.006 g/ml.

