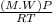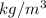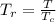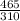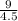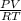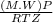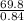Chemistry, 19.03.2020 07:58 kelseiroll9759
A certain gas has a molecular weight of 30.0, a critical temperature of 310 K, and a critical pressure of 4.5 MPa. Calculate the density in kg / m 3 kg/m3 of this gas at 465 K and 9.0 MPa (a) if the gas is ideal and (b) if the gas obeys the law of corresponding states

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2


Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
A certain gas has a molecular weight of 30.0, a critical temperature of 310 K, and a critical pressu...
Questions

History, 06.11.2020 20:50

Chemistry, 06.11.2020 20:50



Mathematics, 06.11.2020 20:50

Chemistry, 06.11.2020 20:50



Mathematics, 06.11.2020 20:50

Mathematics, 06.11.2020 20:50

Business, 06.11.2020 20:50

Mathematics, 06.11.2020 20:50


Mathematics, 06.11.2020 20:50



Mathematics, 06.11.2020 20:50

Physics, 06.11.2020 20:50

English, 06.11.2020 20:50