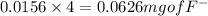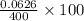A 0.400-g sample of toothpaste was boiled with a 50-mL solution containing a citrate buffer and NaCl to extract a fluoride ion. After cooling, the solution was diluted to exactly 100 mL. The potential of ISE with Ag-AgCl (sat) reference electrode in a 25.0-mL aliquote of the sample was found to be -0.1823 V. Addition of 5.0 mL of a solution containing 0.00107 mg F-/mL caused the potential to change to -0.2446 V. Calculate the percentage of F- in the sample.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
A 0.400-g sample of toothpaste was boiled with a 50-mL solution containing a citrate buffer and NaCl...
Questions


English, 27.05.2020 21:04









Biology, 27.05.2020 21:04


Computers and Technology, 27.05.2020 21:04


English, 27.05.2020 21:04





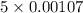 = 0.0053 mg)
= 0.0053 mg) 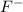 potential will change into -0.2446 V. Hence, potential created by 0.00535 mg
potential will change into -0.2446 V. Hence, potential created by 0.00535 mg 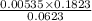 )
)