
Chemistry, 19.03.2020 08:27 AllyJungkookie
A chemist makes a solution of Mg(NO3)2 by dissolving 21.3 g Mg(NO3)2 in water to make 100.0 mL of solution. What is the concentration of NO3− ions in the solution? Assume that Mg(NO3)2 is the only solute in the solution. The molar mass of Mg(NO3)2 is 148.33 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
A chemist makes a solution of Mg(NO3)2 by dissolving 21.3 g Mg(NO3)2 in water to make 100.0 mL of so...
Questions

Mathematics, 16.04.2021 17:10

Physics, 16.04.2021 17:10

Computers and Technology, 16.04.2021 17:10



Spanish, 16.04.2021 17:10


Mathematics, 16.04.2021 17:10

Chemistry, 16.04.2021 17:10

History, 16.04.2021 17:10

Mathematics, 16.04.2021 17:10


Mathematics, 16.04.2021 17:10


Mathematics, 16.04.2021 17:10

Computers and Technology, 16.04.2021 17:10

Mathematics, 16.04.2021 17:10



Computers and Technology, 16.04.2021 17:10

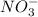 is, 2.88 M
is, 2.88 M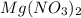 = 21.3 g
= 21.3 g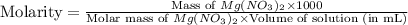
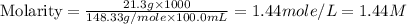
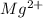 ion and 2 mole of
ion and 2 mole of 

