Chemistry, 19.03.2020 08:57 cristianTalonzo
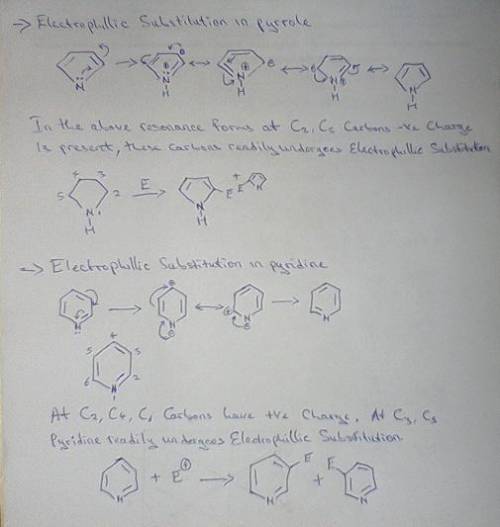
Both pyrrole and pyridine are aromatic compounds, and undergo electrophilic aromatic substitution (EAS). Using a resonance argument, predict the regiochemistry of EAS on both pyrrole and pyridine. As part of your argument, you must draw resonance structures for the arenium ion leading to each possible regiochemistry, and compare the stabilities of the resonance hybrids. In addition, make a reasoned statement about whether you think each one would react faster or slower than benzene and why.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

You know the right answer?
Both pyrrole and pyridine are aromatic compounds, and undergo electrophilic aromatic substitution (E...
Questions


Mathematics, 06.04.2021 04:40


Mathematics, 06.04.2021 04:40

Mathematics, 06.04.2021 04:40



English, 06.04.2021 04:40

Social Studies, 06.04.2021 04:40


Computers and Technology, 06.04.2021 04:40



English, 06.04.2021 04:40



English, 06.04.2021 04:40

History, 06.04.2021 04:40


History, 06.04.2021 04:40