
Chemistry, 19.03.2020 08:58 jetblackcap
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) K c = 1.80 at 250 ∘ C A 0.1846 mol sample of PCl 5 ( g ) is injected into an empty 2.55 L reaction vessel held at 250 ∘ C. Calculate the concentrations of PCl 5 ( g ) and PCl 3 ( g ) at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g...
Questions




Mathematics, 12.11.2020 21:30

Arts, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30

Biology, 12.11.2020 21:30

Biology, 12.11.2020 21:30

History, 12.11.2020 21:30

Social Studies, 12.11.2020 21:30

SAT, 12.11.2020 21:30

Mathematics, 12.11.2020 21:30

Biology, 12.11.2020 21:30



Arts, 12.11.2020 21:30


Mathematics, 12.11.2020 21:30

![[PCl_3]_{eq}=0.0697M\\](/tpl/images/0553/8714/e4a7f.png)
![[PCl_5]_{eq}=0.00269M](/tpl/images/0553/8714/6813e.png)
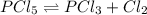
![[PCl_5]_0=\frac{0.1846mol}{2.55L}=0.0724M](/tpl/images/0553/8714/c9006.png)
![Kc=\frac{[Cl_2]_{eq}[PCl_3]_{eq}}{[PCl_5]_{eq}}](/tpl/images/0553/8714/38534.png)
 due to the reaction extent, it becomes:
due to the reaction extent, it becomes: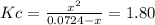
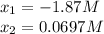
![[PCl_3]_{eq}=x=0.0697M\\](/tpl/images/0553/8714/b7f0d.png)
![[PCl_5]_{eq}=0.0724M-x=0.0724M-0.0697M=0.00269M](/tpl/images/0553/8714/fc102.png)


