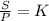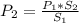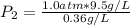2. A gas has a solubility in water
at 0°C of 3.6 g/L at a pressure
of 1.0 atm. What pressu...

Chemistry, 19.03.2020 09:05 queenkimm26
2. A gas has a solubility in water
at 0°C of 3.6 g/L at a pressure
of 1.0 atm. What pressure is
needed to produce an aqueous
solution containing 9.5 g/L of
the same gas at 0°C?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Questions




SAT, 14.12.2020 22:20



Mathematics, 14.12.2020 22:20




Mathematics, 14.12.2020 22:20


Social Studies, 14.12.2020 22:20

Health, 14.12.2020 22:20


Mathematics, 14.12.2020 22:20


Mathematics, 14.12.2020 22:20

Biology, 14.12.2020 22:20

Social Studies, 14.12.2020 22:20