
Chemistry, 19.03.2020 17:16 jadielmatmat
Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it obeys this rate law. rate
rate= (46.6M^-1. s^-1) [H3PO4]^2
Suppose a vessel contains H3PO4 at a concentration of 0.660M. Calculate how long it takes for the concentration of H3PO$ to decrease to 20% to its natural value. You may assume no other reaction is important. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it ob...
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it ob...
Questions




History, 14.01.2020 05:31


Chemistry, 14.01.2020 05:31














![Rate=k[H_3PO_4]^2](/tpl/images/0554/2402/79104.png)
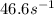
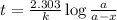
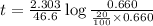
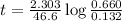
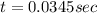
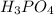 to decrease to 20% to its natural value is 0.0345 sec
to decrease to 20% to its natural value is 0.0345 sec


