
Chemistry, 19.03.2020 19:26 austinmontgomep7foxp
The following two reactions are important in the blast furnace production of iron metal from iron ore (Fe2O3):2C(s) + O2(g) -> 2CO(g)Fe2O3 + 3CO(g) -> 2Fe + 3CO2Using these balanced reactions, how many moles of O2 are required for the production of 5.00 kg of Fe?A) 67.1 molesB) 29.8 molesC) 7.46 molesD) 89.5 molesE) 16.8 moles

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
The following two reactions are important in the blast furnace production of iron metal from iron or...
Questions

Mathematics, 29.10.2021 14:00



Mathematics, 29.10.2021 14:00

History, 29.10.2021 14:00

SAT, 29.10.2021 14:00

SAT, 29.10.2021 14:00

Mathematics, 29.10.2021 14:00

Mathematics, 29.10.2021 14:00

Geography, 29.10.2021 14:00

Mathematics, 29.10.2021 14:00



Social Studies, 29.10.2021 14:00



Biology, 29.10.2021 14:00


Mathematics, 29.10.2021 14:00

Medicine, 29.10.2021 14:00

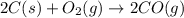
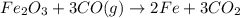
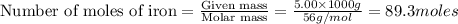
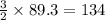 moles of carbon monoxide
moles of carbon monoxide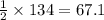 moles of oxygen
moles of oxygen

