
Chemistry, 19.03.2020 21:10 hardwick744
Find the number of moles of water that can be formed if you have 170 mol of hydrogen gas and 80 mol of oxygen gas. Express your answer with the appropriate units.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2
You know the right answer?
Find the number of moles of water that can be formed if you have 170 mol of hydrogen gas and 80 mol...
Questions

Spanish, 09.07.2019 05:00

Biology, 09.07.2019 05:00

Mathematics, 09.07.2019 05:00



Computers and Technology, 09.07.2019 05:00

Chemistry, 09.07.2019 05:00

Computers and Technology, 09.07.2019 05:00

Computers and Technology, 09.07.2019 05:00

Mathematics, 09.07.2019 05:00





Mathematics, 09.07.2019 05:00

Computers and Technology, 09.07.2019 05:00




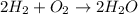
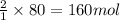 of hydrogen gas
of hydrogen gas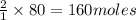 of water
of water

