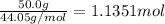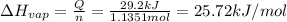29.2 kJ of heat is required to convert an 50.0 g sample of
acetaldehyde from the liquid to gas...

Chemistry, 19.03.2020 20:37 rexerlkman4145
29.2 kJ of heat is required to convert an 50.0 g sample of
acetaldehyde from the liquid to gas phase. The molecular
weight of acetaldehyde is 44.05 g/mol. What is the heat of
vaporization of acetaldehyde in kJ/mol?
kJ/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Questions

Mathematics, 25.01.2022 01:00




History, 25.01.2022 01:00


Mathematics, 25.01.2022 01:00

English, 25.01.2022 01:00


History, 25.01.2022 01:00

Mathematics, 25.01.2022 01:00



Mathematics, 25.01.2022 01:00

English, 25.01.2022 01:00



Mathematics, 25.01.2022 01:00

Chemistry, 25.01.2022 01:00