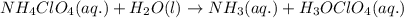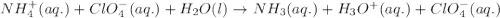Chemistry, 19.03.2020 20:29 lalaokawami0912
Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium perchlorate is dissolved in water. (Use H3O instead of H .)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2


Chemistry, 23.06.2019 13:30
Did you mention that adding weight increased the pressure
Answers: 2
You know the right answer?
Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammon...
Questions

English, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50


Social Studies, 18.12.2020 02:50

Chemistry, 18.12.2020 02:50


Business, 18.12.2020 02:50

Spanish, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Chemistry, 18.12.2020 02:50




Mathematics, 18.12.2020 02:50

Physics, 18.12.2020 02:50


Spanish, 18.12.2020 02:50


English, 18.12.2020 02:50

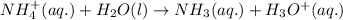
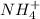 and
and 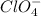 ions.
ions. 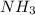 .
. 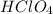 .
.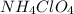 ,
, 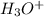 .
.