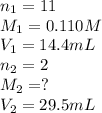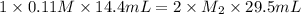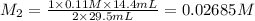Chemistry, 19.03.2020 20:29 adhitrfbvg
An aqueous solution of barium hydroxide is standardized by titration with a 0.110 M solution of hydrochloric acid. If 29.5 mL of base are required to neutralize 14.4 mL of the acid, what is the molarity of the barium hydroxide solution

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.110 M solution of hydr...
Questions


Mathematics, 04.07.2020 01:01

Mathematics, 04.07.2020 01:01

Mathematics, 04.07.2020 01:01



Computers and Technology, 04.07.2020 01:01




Health, 04.07.2020 01:01


Chemistry, 04.07.2020 01:01

Computers and Technology, 04.07.2020 01:01






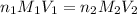
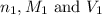 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 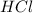
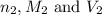 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 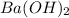 mm.
mm.