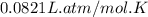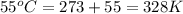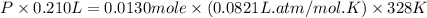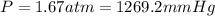Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
What is the pressure in millimeters of mercury of 0.0130 molmol of helium gas with a volume of 210....
Questions

History, 17.10.2020 09:01


Mathematics, 17.10.2020 09:01

Mathematics, 17.10.2020 09:01





Health, 17.10.2020 09:01

English, 17.10.2020 09:01



Biology, 17.10.2020 09:01


Computers and Technology, 17.10.2020 09:01

History, 17.10.2020 09:01

Biology, 17.10.2020 09:01


Mathematics, 17.10.2020 09:01

Mathematics, 17.10.2020 09:01

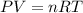
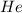 gas = ?
gas = ?