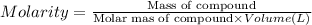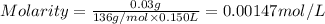Chemistry, 19.03.2020 20:54 sonyarucker
A chemist prepares a solution of calcium sulfate CaSO4 by measuring out 0.03g of calcium sulfate into a 150.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's calcium sulfate solution. Be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
A chemist prepares a solution of calcium sulfate CaSO4 by measuring out 0.03g of calcium sulfate int...
Questions


Biology, 05.04.2020 02:47




Mathematics, 05.04.2020 02:48

Mathematics, 05.04.2020 02:48

Mathematics, 05.04.2020 02:48


Business, 05.04.2020 02:48

Mathematics, 05.04.2020 02:48






Mathematics, 05.04.2020 02:48

Biology, 05.04.2020 02:48

Mathematics, 05.04.2020 02:48

English, 05.04.2020 02:48