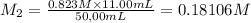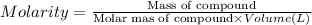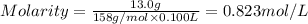Chemistry, 19.03.2020 20:56 Uhmjujiooo45701
By pipet, 11.00 mL of a 0.823 MM stock solution of potassium permanganate (KMnO4) was transferred to a 50.00-mL volumetric flask and diluted to the calibration mark. Determine the molarity of the resulting solution. A stock solution of potassium permanganate (KMnO4) was prepared by dissolving 13.0g KMnO4 with DI H2O in a 100.00-mL volumetric flask and diluting to the calibration mark. Determine the molarity of the solution Molarity= O.822 M

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


You know the right answer?
By pipet, 11.00 mL of a 0.823 MM stock solution of potassium permanganate (KMnO4) was transferred to...
Questions

Mathematics, 10.12.2021 17:50


Biology, 10.12.2021 17:50

English, 10.12.2021 17:50

Mathematics, 10.12.2021 17:50



Computers and Technology, 10.12.2021 17:50

History, 10.12.2021 17:50

Physics, 10.12.2021 17:50

Mathematics, 10.12.2021 17:50

Physics, 10.12.2021 17:50





Mathematics, 10.12.2021 17:50

Mathematics, 10.12.2021 17:50


English, 10.12.2021 17:50

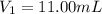
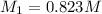
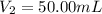
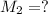
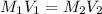 ( dilution )
( dilution )