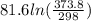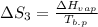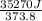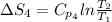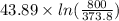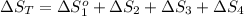The standard entropy of liquid methanol at 298K is 126.8 J/K-mol and its heat capacity is 81.6 J/K-mol. Methanol boils at 337K with an enthalpy of vaporization of 35.270 kJ/mol at that temperature. The heat capacity of the vapor is 43.9 J/K-mol.__Calculate the entropy of one mole of methanol vapor at 800 K.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
The standard entropy of liquid methanol at 298K is 126.8 J/K-mol and its heat capacity is 81.6 J/K-m...
Questions

Mathematics, 17.12.2020 21:40

Mathematics, 17.12.2020 21:40



Chemistry, 17.12.2020 21:40


Physics, 17.12.2020 21:40

Mathematics, 17.12.2020 21:40




Mathematics, 17.12.2020 21:40


Computers and Technology, 17.12.2020 21:40



Mathematics, 17.12.2020 21:40

Mathematics, 17.12.2020 21:40


Biology, 17.12.2020 21:40

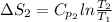 J/K mol
J/K mol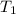 = 298 K,
= 298 K, 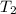 = 373.8 K
= 373.8 K