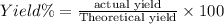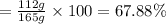Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 10:30
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
You know the right answer?
When 60.0 g of CH4 reacts with excess O2, the actual yield of CO2 is 112 g. What is the percent yiel...
Questions

English, 06.04.2020 18:09


History, 06.04.2020 18:09


Mathematics, 06.04.2020 18:09




Computers and Technology, 06.04.2020 18:09

Computers and Technology, 06.04.2020 18:09

Mathematics, 06.04.2020 18:09




Geography, 06.04.2020 18:09





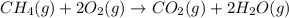
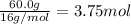
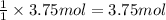 of carbon dioxide gas
of carbon dioxide gas