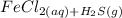Chemistry, 19.03.2020 21:27 Hamadsaqer9
Solid iron(II) sulfide reacts with aqueous hydrochloric acid HCl to produce hydrogen sulfide gas H2S and aqueous iron(II) chloride . Write a balanced chemical equation for this reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1


Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
Solid iron(II) sulfide reacts with aqueous hydrochloric acid HCl to produce hydrogen sulfide gas H2S...
Questions

Computers and Technology, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01










Mathematics, 09.09.2020 23:01




Computers and Technology, 09.09.2020 23:01


Mathematics, 09.09.2020 23:01


History, 09.09.2020 23:01