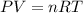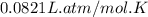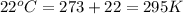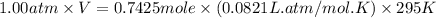Chemistry, 19.03.2020 21:25 xemnas1994
Automobile airbags contain solid sodium azide, NaN3,that reacts to produce nitrogen gas when heated, thus inflating the bag.2NaN3(s)⟶2Na(s)+3N2(g)Calculate the value of work, with, for the system if32.2NaNO3 reacts completely at1.00 atmand22∘C.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Automobile airbags contain solid sodium azide, NaN3,that reacts to produce nitrogen gas when heated,...
Questions


Mathematics, 05.05.2020 21:13

Advanced Placement (AP), 05.05.2020 21:13








Chemistry, 05.05.2020 21:14



Biology, 05.05.2020 21:14

Mathematics, 05.05.2020 21:14


Mathematics, 05.05.2020 21:14



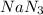
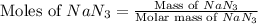
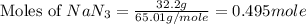
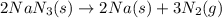
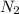
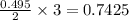 moles of
moles of