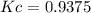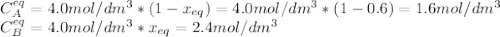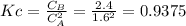The elementary reversible reaction:
2A <> B
is carried out isotherma...

The elementary reversible reaction:
2A <> B
is carried out isothermally and isobarically in a flow reactor where pure A is fed at a concentration of 4.0 mol/dm3. If the equilibrium conversion is found to be 60%. What is the equilibrium constant, Kc if the reaction is a gas phase reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1


Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
Questions

Computers and Technology, 18.12.2021 21:10

Biology, 18.12.2021 21:10


Social Studies, 18.12.2021 21:10

Mathematics, 18.12.2021 21:10


English, 18.12.2021 21:10


Geography, 18.12.2021 21:20

Social Studies, 18.12.2021 21:20



Mathematics, 18.12.2021 21:20


History, 18.12.2021 21:20

Mathematics, 18.12.2021 21:20

Mathematics, 18.12.2021 21:20


History, 18.12.2021 21:20