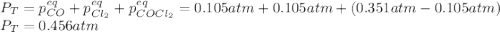CO(g) + Cl2 (g) ⇌ COCl2 (g) Kp = 22.5 at 395 °C . A sample of COCl2 (g) is introduced into a constantvolume vessel at 395 °C and observed to exert an initial pressure of 0.351 atm. When equilibrium is established, what will be the total pressure within the vessel?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

You know the right answer?
CO(g) + Cl2 (g) ⇌ COCl2 (g) Kp = 22.5 at 395 °C . A sample of COCl2 (g) is introduced into a constan...
Questions




Biology, 24.06.2019 15:00


Biology, 24.06.2019 15:00

Physics, 24.06.2019 15:00








Mathematics, 24.06.2019 15:00

Mathematics, 24.06.2019 15:00




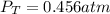
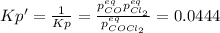
 due to reaction's progress, one obtains:
due to reaction's progress, one obtains: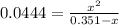
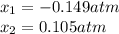
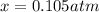 for which the total pressure at equilibrium is:
for which the total pressure at equilibrium is: