
Chemistry, 19.03.2020 22:32 ogjigglypuff
Predict which of the following will be formed at the anode in the electrolysis of an aqueous solution of KI.
a. KI.
b. K
c. O2
d. H2
e. I2

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2
You know the right answer?
Predict which of the following will be formed at the anode in the electrolysis of an aqueous solutio...
Questions







History, 14.01.2020 20:31


Spanish, 14.01.2020 20:31




Biology, 14.01.2020 20:31


Physics, 14.01.2020 20:31

Mathematics, 14.01.2020 20:31

History, 14.01.2020 20:31


Computers and Technology, 14.01.2020 20:31

Chemistry, 14.01.2020 20:31

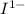 ) is oxidized to I2 at the anode. One can see by the
) is oxidized to I2 at the anode. One can see by the


