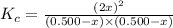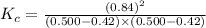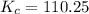Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2. An equilibrium reaction e...
Questions





English, 30.06.2019 04:00


Mathematics, 30.06.2019 04:00



Chemistry, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00


Mathematics, 30.06.2019 04:00


Mathematics, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00



Mathematics, 30.06.2019 04:00

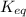 is 110.25
is 110.25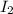 = 0.500 mole
= 0.500 mole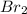 = 0.500 mole
= 0.500 mole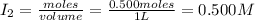
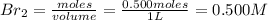
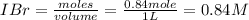 [/tex]
[/tex]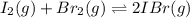
![K_c=\frac{[IBr]^2}{[Br_2]\times [I_2]}](/tpl/images/0554/7930/da4ed.png)