Chemistry, 19.03.2020 23:34 roseemariehunter12
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia:
N2(g) + 3H2(g) > 2NH3(g) H = -92.kJ
In the second step, ammonia and oxygen react to form nitric acid and water:
NH3(g) + 2O2(g) > HNO3(g) + H20 (g) H=-330.kJ
Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen. Round your answer to the nearest kJ.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

You know the right answer?
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen...
Questions

English, 04.02.2020 06:55

Mathematics, 04.02.2020 06:55

History, 04.02.2020 06:55






Social Studies, 04.02.2020 06:55

History, 04.02.2020 06:55

Biology, 04.02.2020 06:55

Mathematics, 04.02.2020 06:55

History, 04.02.2020 06:55





Chemistry, 04.02.2020 06:55


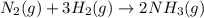 (1)
(1)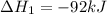
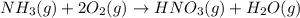 (2)
(2)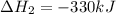
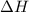 for the following reaction i.e,
for the following reaction i.e,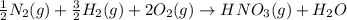
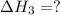 (3)
(3)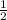 and add to 2.
and add to 2.