
Chemistry, 19.03.2020 23:13 esmeralda266
Chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250.mL sample of groundwater known to be contaminated with iron(III) chloride, which would react with silver nitrate solution like this:FeCl3(aq) + 3AgNO3(aq) ⟶ 3AgCl(s) + FeNO3(aq)The chemist adds 82.0 M silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 2.5mg of silver chloride. Calculate the concentration of iron(III) chloride contaminant in the original groundwater sample.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Chloride anions in solution will combine with the silver cations to produce bright white silver chlo...
Questions

Mathematics, 23.07.2019 18:30





Mathematics, 23.07.2019 18:30


History, 23.07.2019 18:30


English, 23.07.2019 18:30



Biology, 23.07.2019 18:30




Mathematics, 23.07.2019 18:30

Mathematics, 23.07.2019 18:30

Mathematics, 23.07.2019 18:30

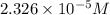 the concentration of iron(III) chloride contaminant in the original groundwater sample.
the concentration of iron(III) chloride contaminant in the original groundwater sample.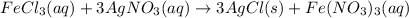
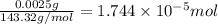
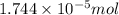 od silver chloride will be obtained from ;
od silver chloride will be obtained from ;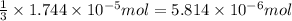 of ferric chloride
of ferric chloride![[FeCl_3]=\frac{5.814\times 10^{-6} mol}{0.250 L}=2.326\times 10^{-5} M](/tpl/images/0554/8189/91194.png)


