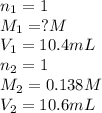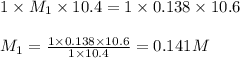Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
An aqueous solution of hydroiodic acid is standardized by titration with a 0.138 M solution of potas...
Questions


Mathematics, 06.06.2021 01:00

English, 06.06.2021 01:00

Chemistry, 06.06.2021 01:00


Mathematics, 06.06.2021 01:00







Biology, 06.06.2021 01:00







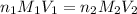
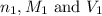 are the n-factor, molarity and volume of acid which is HI
are the n-factor, molarity and volume of acid which is HI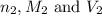 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.