
Chemistry, 19.03.2020 23:33 littledudefromacross
A sample of helium (He) gas initially at 19°C and 1.0 atm is expanded from 1.4 L to 2.8 L and simultaneously heated to 35°C. Calculate the entropy change for the process.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


Chemistry, 23.06.2019 13:30
Which correctly identifies the parts of a transverse wave? a: crest b: amplitude c: wavelength d: trough a: trough b: amplitude c: crest d: wavelength a: trough b: amplitude c: wavelength d: crest a: crest b: amplitude c: trough d: wavelength
Answers: 1
You know the right answer?
A sample of helium (He) gas initially at 19°C and 1.0 atm is expanded from 1.4 L to 2.8 L and simult...
Questions

Computers and Technology, 23.09.2020 14:01

History, 23.09.2020 14:01


History, 23.09.2020 14:01



Mathematics, 23.09.2020 14:01




Mathematics, 23.09.2020 14:01


Spanish, 23.09.2020 14:01




Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


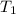 = (19 + 273) K = 292 K,
= (19 + 273) K = 292 K, 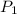 = 1.0 atm,
= 1.0 atm,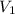 = 1.4 L
= 1.4 L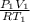
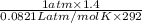

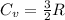
 ) of 2.8 L and it is heated to
) of 2.8 L and it is heated to 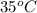 or (35 + 273) K = 308 K.
or (35 + 273) K = 308 K.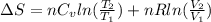
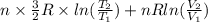
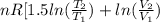
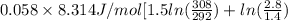
![0.4822 \times [1.5 \times 0.052 + 0.693]](/tpl/images/0554/8697/09f71.png)


