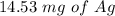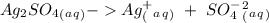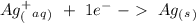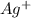Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
Suppose a current of 250.mA is passed through an electroplating cell with an aqueous solution of Ag2...
Questions

Mathematics, 23.12.2020 15:00

History, 23.12.2020 15:00

Mathematics, 23.12.2020 15:00

Mathematics, 23.12.2020 15:00

Social Studies, 23.12.2020 15:10

History, 23.12.2020 15:10

Mathematics, 23.12.2020 15:10


Mathematics, 23.12.2020 15:20


English, 23.12.2020 15:20

Mathematics, 23.12.2020 15:20

Mathematics, 23.12.2020 15:20

Law, 23.12.2020 15:20


History, 23.12.2020 15:20


History, 23.12.2020 15:20

English, 23.12.2020 15:20

Mathematics, 23.12.2020 15:20