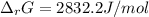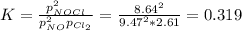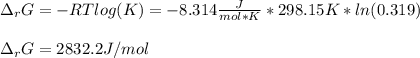Chemistry, 20.03.2020 00:06 nguyenhoangthienkim0
A chemist fills a reaction vessel with 9.47 atm nitrogen monoxide (NO) gas, 2.61 atm chlorine (C12) gas, and 8.64 atm nitrosyl chloride (NOCI) gas at a temperature of 25.0°C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction:
2NO(g) + Cl2(g) <=> 2NOCI (g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
A chemist fills a reaction vessel with 9.47 atm nitrogen monoxide (NO) gas, 2.61 atm chlorine (C12)...
Questions

Mathematics, 28.11.2020 04:40


Mathematics, 28.11.2020 04:40


Business, 28.11.2020 04:40


Mathematics, 28.11.2020 04:40

Mathematics, 28.11.2020 04:40

Mathematics, 28.11.2020 04:40










Computers and Technology, 28.11.2020 04:40