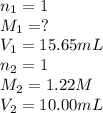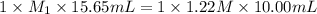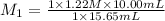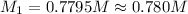Chemistry, 19.03.2020 23:59 michael1498
In a titration, 15.65 milliliters of a KOH * (aq) ) solution exactly neutralized 10.00 milliliters of a 1.22 M HCl * (aq) solution . In the space below , show a correct numerical setup for calculating the molarity of the KOH * (aq) solution .

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
How many joules of heat are absorbed to raise the temperature of 650 grams of water from 5.00c to it's boiling point, 100c
Answers: 1

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

You know the right answer?
In a titration, 15.65 milliliters of a KOH * (aq) ) solution exactly neutralized 10.00 milliliters o...
Questions


Biology, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Social Studies, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Health, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30


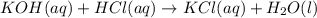
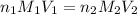
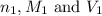 are the n-factor, molarity and volume of acid which is KOH
are the n-factor, molarity and volume of acid which is KOH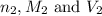 are the n-factor, molarity and volume of base which is HCl.
are the n-factor, molarity and volume of base which is HCl.