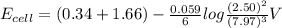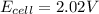Chemistry, 19.03.2020 23:58 naseersaad
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: →+3Cu+2aq2Als+3Cus2Al+3aq Suppose the cell is prepared with 7.97 M Cu+2 in one half-cell and 2.50 M Al+3 in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: →+3Cu+2aq2Als...
Questions




Health, 28.08.2019 03:30

Biology, 28.08.2019 03:30

Biology, 28.08.2019 03:30


Spanish, 28.08.2019 03:30




Biology, 28.08.2019 03:30


Mathematics, 28.08.2019 03:30

Mathematics, 28.08.2019 03:30


Mathematics, 28.08.2019 03:30

History, 28.08.2019 03:30


Mathematics, 28.08.2019 03:30

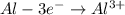 )
)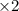 ;
; 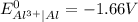
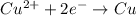 )
)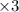 ;
; 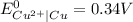
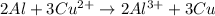
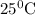 -
-![E_{cell}=[E_{Cu^{2+}\mid Cu}^{0}-E_{Al^{3+}\mid Al}^{0}]-\frac{0.059}{n}log\frac{[Al^{3+}]^{2}}{[Cu^{2+}]^{3}}](/tpl/images/0554/9233/c4d3d.png)