
Chemistry, 19.03.2020 23:58 johnnydenali67
A 2.45-g sample of strontium completely reacts with oxygen to form 2.89 g of strontium oxide. Use this data to calculate the mass percent composition of strontium in strontium oxide.

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 11:40
Which of the following would have the lowest average kinetic energy
Answers: 1

Chemistry, 23.06.2019 19:30
(04.03 hc) what type of energy transformation takes place during cellular respiration? use complete sentences to explain how energy is conserved during cellular respiration.
Answers: 1

Chemistry, 23.06.2019 23:10
How is temperature related to the physical change of a substance?
Answers: 1
You know the right answer?
A 2.45-g sample of strontium completely reacts with oxygen to form 2.89 g of strontium oxide. Use th...
Questions

Spanish, 05.03.2021 17:30





Advanced Placement (AP), 05.03.2021 17:30



Physics, 05.03.2021 17:30

Mathematics, 05.03.2021 17:30


English, 05.03.2021 17:30

English, 05.03.2021 17:30


Chemistry, 05.03.2021 17:30

Physics, 05.03.2021 17:30


Mathematics, 05.03.2021 17:30


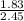 × 100
× 100 

