Chemistry, 20.03.2020 00:04 janessa0804
Find the mass of AlCl3 that is produced when 10.0 grams of Al2O3 react with 10.0 g of HCl according to the following equation. Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2


Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

You know the right answer?
Find the mass of AlCl3 that is produced when 10.0 grams of Al2O3 react with 10.0 g of HCl according...
Questions















Mathematics, 04.06.2020 19:08

Mathematics, 04.06.2020 19:08




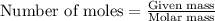 .....(1)
.....(1)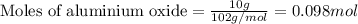
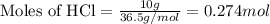
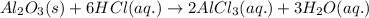
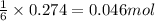 of aluminium oxide
of aluminium oxide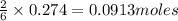 of aluminium chloride
of aluminium chloride