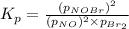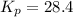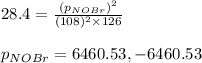Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1


Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
Consider the reaction: 2 NO(g) + Br2(g) ∆ 2 NOBr(g) Kp = In a reaction mixture at equilibrium, the p...
Questions





Mathematics, 06.09.2020 02:01

Mathematics, 06.09.2020 02:01


Computers and Technology, 06.09.2020 02:01

English, 06.09.2020 02:01

Mathematics, 06.09.2020 02:01

Mathematics, 06.09.2020 02:01

English, 06.09.2020 02:01

Mathematics, 06.09.2020 02:01

Chemistry, 06.09.2020 02:01

Biology, 06.09.2020 02:01


Mathematics, 06.09.2020 02:01


Mathematics, 06.09.2020 02:01

Mathematics, 06.09.2020 02:01

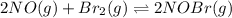
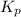 for above equation follows:
for above equation follows: