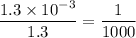Chemistry, 20.03.2020 01:06 blondielocks2002
Explain why a solution that is 1.3 M HF and 1.3 mM KF is not a good buffer. HF is a strong acid and cannot be used in a buffer system. The ratio of acid to conjugate base is outside the buffer range of 10:1. The two species are not a conjugate acid base pair. KF is not soluble in water and cannot be used in a buffer system.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
Explain why a solution that is 1.3 M HF and 1.3 mM KF is not a good buffer. HF is a strong acid and...
Questions


SAT, 15.12.2020 01:20

Physics, 15.12.2020 01:20


Computers and Technology, 15.12.2020 01:20



Biology, 15.12.2020 01:20

Biology, 15.12.2020 01:20

Mathematics, 15.12.2020 01:20

History, 15.12.2020 01:20



Chemistry, 15.12.2020 01:20



Mathematics, 15.12.2020 01:20



Mathematics, 15.12.2020 01:20

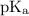 + log
+ log 
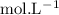 in HF and 1.3
in HF and 1.3 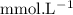 in KF, the ratio is:
in KF, the ratio is: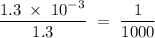
![\text{pH} = \text{pK}_{\text{a}} + \log\dfrac{\text{[A$^{-}$]}}{\text{[HA]}}](/tpl/images/0555/0502/0e377.png)
![\dfrac{1}{10} \leq \dfrac{\text{[A$^{-}]$}}{\text{[HA]}} \leq \dfrac{10}{1}](/tpl/images/0555/0502/63cff.png)